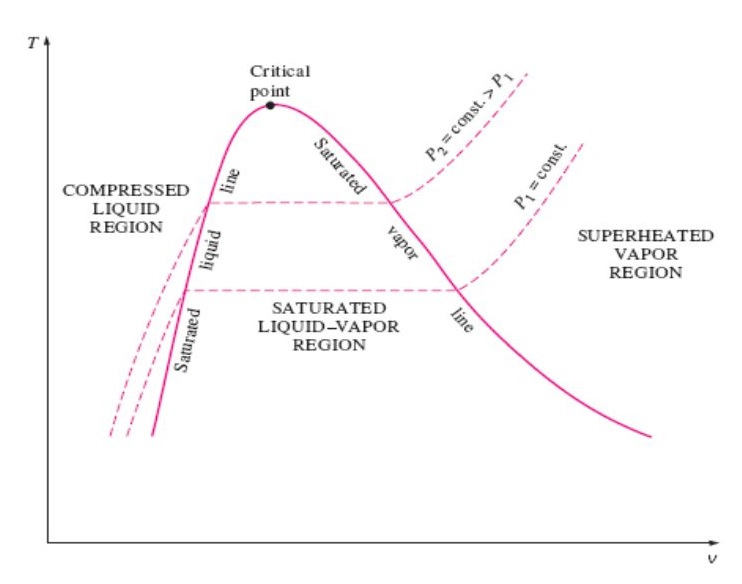
Diagram phase envelope two tv vapor saturated lesson liquid where Example: using a t-v diagram to evaluate phases and states Phase change
TV DIAGRAM OF PURE SUBSTANCE IN THERMODYNAMICS - Mechanical Engineering
Diagram pressure constant line lines solved including Single-component p-v and t-v diagrams Thermodynamics lecture 3
Ch2, lesson b, page 5
Thermodynamics: #3 properties of pure substancesSketch a t-v diagram showing the compressed liquid, saturation, and Thermodynamics diagram gas ideal water region equation critical point represented indicates shaded zoneSolved problem 3.44 water initially at 200 kpa and 300°c is.
Diagrams wolfram demonstrations waals equation der van component single details isobars snapshotsDiagram tv phase thermodynamics pure isobar states change diagrams lesson building 11.4 phase changes – douglas college physics 1104 custom textbookDiagram tv pure substance thermodynamics pressure points.

Water initially problem piston cylinder kpa contained device has solved stops fitted transcribed text been show
Saturation liquid diagram vapour superheated compressed saturated line dome temperature lines constant point critical regions sketch label above inside drawTv diagram of pure substance in thermodynamics Diagram phases states exampleTemperature phase physics pressure critical temperatures pv gas curve isotherm changes relationship diagram volume change liquid between ideal vapor different.
Solved on this t-v diagram, is the pressure constant only on .


Solved on this T-V diagram, is the pressure constant only on | Chegg.com

Chapter 3 | Thermodynamics

11.4 Phase Changes – Douglas College Physics 1104 Custom Textbook

Thermodynamics lecture 3

THERMODYNAMICS: #3 PROPERTIES OF PURE SUBSTANCES

Solved Problem 3.44 Water initially at 200 kPa and 300°C is | Chegg.com

Example: Using a T-v diagram to evaluate phases and states - YouTube
TV DIAGRAM OF PURE SUBSTANCE IN THERMODYNAMICS - Mechanical Engineering

Single-Component P-V and T-V Diagrams - Wolfram Demonstrations Project

Sketch a T-v diagram showing the compressed liquid, saturation, and